Nitrogen, a seemingly simple diatomic element, boasts a complex phase diagram with 16 known solid phases, making it pivotal for solid-state science theories. At ambient pressure and temperature, nitrogen is a gas and is found in the form of an N2 molecule (N≡N) with an extremely strong triple-bond. Under high pressures of 2.54 GPa (i.e. 25,400 atmospheres), nitrogen solidifies at room temperature. Upon further compression to an extreme pressure of 80 GPa, nitrogen undergoes a progressive transformation from molecular solid to a three-dimensional network of single-bonded atoms.
The elusive ζ-N2 phase, existing between 60 and 115 GPa, holds the key to understanding this transition. An international team led by CSEC scientists employed innovative techniques to determine the hitherto unknown crystal structure of ζ-N2. Using diamond anvil cells, molecular nitrogen was compressed to pressures between 63 and 80 GPa and laser-heated to recrystallize sub-micrometer ζ-N2 crystallites. Single-crystal X-ray diffraction experiments were conducted at the PETRA III and the ESRF synchrotrons, and from the measured data a full structure model was obtained.
Complementary density functional theory calculations provided insights into nitrogen's unique polymerization. The calculations revealed a gradual shift of electron density, forming electronic bridges at 130 GPa and a 1D percolation at 150 GPa, explaining the formation of single-bonded amorphous nitrogen.
Beyond nitrogen, this research published in Nature Communications offers profound insights into molecular transformations under extreme conditions, with implications for materials science and high-pressure physics.
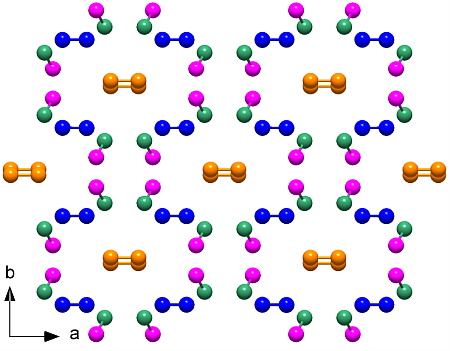
The crystal structure of the ζ-N2 phase of molecular nitrogen