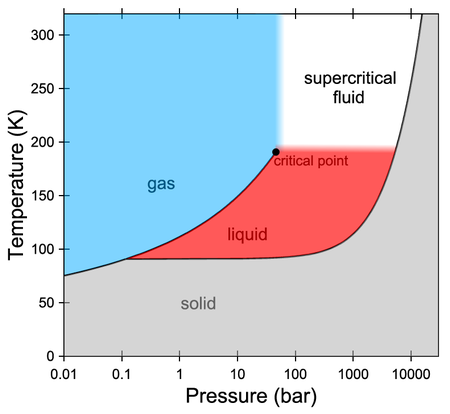
A liquid or gaseous substance pushed beyond its critical point (i.e. beyond the temperature and pressure at which the distinction between liquid and gas can no longer be made) is called a supercritical fluid. Still little-known and defying conventional classifications, supercritical fluids possess the ability to effuse like a gas while dissolving materials like a liquid. This duality has made them invaluable in myriads of industrial applications, from pharmaceutical processing to waste treatment. On the other hand, they are crucial to understanding giant planets such as Jupiter, where such a state of matter may reign.
In a study now published in Nature Communications, an international collaboration including the Centre for Science at Extreme Conditions (CSEC) at the University of Edinburgh obtained experimental proof that the behaviour of supercritical fluid methane can be either gas-like or liquid-like. Specifically, the researchers investigated the molecular diffusion coefficient – a crucial parameter reflecting the mobility of the molecules in the sample – with a fundamental question in mind: can we pinpoint a region of the supercritical fluid state where the diffusion mechanism changes from gas-like to liquid-like?
For this challenging experimental work, quasi-elastic neutron scattering measurements on supercritical fluid methane were conducted at the Institut Laue-Langevin in Grenoble, France. The measurements took place at a constant temperature of 200 K (just above the critical temperature of 191 K) while varying the pressure of the sample from a few bars up to nearly 2500 bar (the critical pressure being 46 bar).
This research offers new insights into molecular behaviour under extreme conditions, with implications spanning from industry to planetary modelling. Also, as often happens in research, having opened a door means seeing new avenues to explore.