Recent research suggests that hydrogen-based solids are among the top contenders for high-temperature superconductors. Since 2015, high-pressure sciences have been at the leading edge of the synthesis of materials with high superconducting critical temperatures (Tc), namely with a breakthrough Tc of 260 K reported in the lanthanum hydride (La-H) system at 1,600,000 times atmospheric pressure. Yet, incongruities in experimental measurements suggested other phases to be formed along with LaH10+δ.
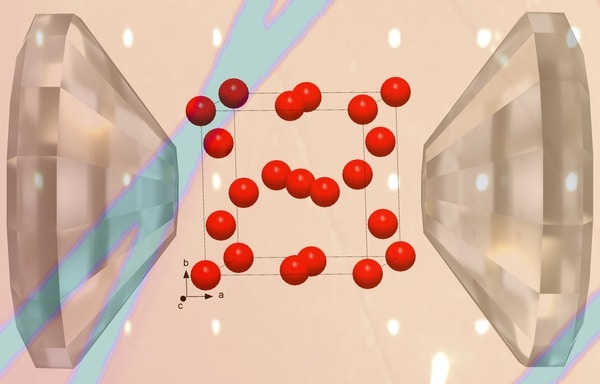
A CSEC-led international team with collaborators from the University of Bayreuth and Linköping University employed extreme pressure and temperature conditions to investigate the La-H system. Employing laser-heated diamond anvil cells, they synthesized at pressures between 96 and 176 GPa a total of seven lanthanum hydrides: five novel compounds — LaH~4, LaH4+δ, La4H23, LaH6+δ and LaH9+δ — as well as the previously known LaH3 and LaH10+δ solids. The atomic arrangement of the non-hydrogen atoms in these solids was then determined at the PETRA III and APS synchrotrons using single-crystal X-ray diffraction.
These results, published in Nature Communications, demonstrated that all of these lanthanum hydrides except LaH~4 have La arrangements identical to those previously observed in other rare-earth–H systems, bringing to light crystal-chemical regularities common for various rare-earth–H systems. Moreover, these results showed that these compounds exhibit a variability of hydrogen content for a given arrangement of the La atoms, underpinning extreme difficulties of DFT calculations to accurately determine the content and the position of the H atoms in these solids. This hydrogen content variability will undoubtedly provide the impetus for revising theoretical models of high-pressure hydrides, ultimately leading to an intimate understanding of their superconductivity and bringing us one step closer to a room-temperature superconductor.