Until very recently, helium had remained the last naturally occurring element that was known not to form stable solid compounds. Dr Andreas Hermann from CSEC and collaborators in China and the USA have now demonstrated that there is a general driving force for helium to react with ionic compounds that contain an unequal number of cations and anions. The corresponding reaction products are stabilized not by local chemical bonds but by long-range Coulomb interactions that are significantly modified by the insertion of helium atoms, especially under high pressure. This mechanism also explains the recently discovered reactivity of He and Na under pressure. The study reveals that helium has the propensity to react with a broad range of ionic compounds at pressures as low as 30 GPa. Since most of the Earth’s minerals contain unequal numbers of positively and negatively charged atoms, their work suggests that large quantities of He might be stored in the Earth’s lower mantle.
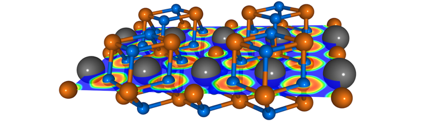
Crystal structure of MgF2He, together with a cross section of the electron localisation function (ELF). Grey/orange/blue spheres show the He/Mg/F atoms. ELF values vary from zero (blue) to one (red).